Around 7:30 p.m. on Tuesday evening, a Domino’s pizza delivery man was attacked as he was making a delivery to a Dallas apartment complex. The address where the delivery was made was a vacant apartment. When he arrived to his destination at the Sienna Palms Apartments in northeast Dallas, the delivery man was immediately attacked. He tried running, but unfortunately, he was gunned down and shot in the back. The delivery man’s condition is still unknown and is currently being treated at Baylor Hospital. The suspect is still at large and police are currently investigating the incident.
Our thoughts and prayers go out to the victim and his family during this tough time.
Borchardt Law Firm currently represents the family of a Domino’s pizza delivery man who was assaulted while making a delivery. Unfortunately, the injuries that were inflicted ended his life. A claim has been brought against the local franchise and the national corporation of Domino’s Pizza alleging that the company failed to implement security policies and procedures on the safe delivery of pizza, particularly in unsafe areas. The attorney on the case, Geno Borchardt, stated, “they have numerous policies in place to protect the safety of their driver, but many of these policies were ignored. The victim of this incident was a good man. He loved his wife deeply and their life together was ruined because Domino’s failed to follow safe procedures. The purpose of this lawsuit is to ensure that nothing this tragic ever happens to anybody else.” (read full story)
FDA Puts Johnson & Johnson Back In The Headlines
A warning letter sent by the U.S. Food and Drug Administration (FDA) puts Johnson & Johnson back in the headlines. On December 8, 2011 the FDA disclosed new information in a warning letter stating one of the company’s subsidiaries sold multiple types of orthopaedic devices with out the required approval from regulators. The letter was based on an inspection of DePuy Orthaepedics from May 10, 2011 to June 7, 2011. The inspection showed DePuy had cleared 14 devices, mostly knee or hip systems, with out receiving proper approval from the FDA.
Unfortunately for Johnson & Johnson, this isn’t the first time for DePuy to make the headlines for negative publicity. In August of 2010 Johnson & Johnson issued a recall for their ASR XL Acetabular Hip Replacement System after receiving hundreds of lawsuits due to the failing device. Before the recall, the company believed the ASR XL Acetabular system was used in more than 90,000 patients, creating a large amount of concern for the majority of those patients.
Since the ASR XL Acetabular recall, Johnson & Johnson has been closely watched by the FDA. With the most recent warning, the company is striving to maintain a positive image. After the warning letter was sent out in December, DePuy responded by stating that the cleared devices were custom-made for certain patients and did not need the regular required approval. However, the FDA argued otherwise stating that, “the fact that the final specifications are tailored to match a patient’s anatomy does not preclude a clinical study or submission of a marketing application for the devices.” The FDA also stated that DePuy failed to obey with quality regulations for the devices. DePuy has currently discontinued all custom-made devices and is trying to answer all questions that raise concern in the warning letter.
—–
Johnson & Johnson View $158 Million Settlement as a Win in Texas Case
Johnson & Johnson, the world’s eighth-largest pharmaceuticals company, has made the news again, unfortunately, not for positive reasons. After the company had to recall their DePuy ASR™ XL Acetabular Hip System and the ASR™ Hip Resurfacing System in 2010, they faced hundreds of lawsuits involving failed medical devices and have continuously received more cases. Though, their most recent lawsuit was for something different.
Risperdal, an anti-psychotic medication, also known as an “atypical anti-psychotic,” is used to treat schizophrenia and symptoms of bipolar disorder. It may also be used to treat symptoms of irritability for autistic children. Risperdal was created by Janssen Pharmaceuticals Inc., a subsidiary of Johnson & Johnson, and has been on the market for several years. After years of patient use, complaints began to pile up claiming Janssen Pharmaceuticals Inc. made false or misleading statements about the safety, cost and effectiveness of the expensive drug. Patients also worried that the pharmaceutical company had been improperly influencing doctors to market the drug. Risperdal is not a cheap drug and has earned Johnson & Johnson close to a billion in profit.
Just last year a South Carolina jury found Johnson & Johnson guilty of overstating the safety and effectiveness of Risperdal, settling the case at $327 million. Shortly after, a Louisiana jury found the company guilty for violating that state’s Medicaid fraud act settling the case at $258 million. When a Texas attorney general heard of the two cases he added up all the money the state’s Medicaid program has spent on Risperdal, the attorney proposed Johnson & Johnson pay $579 million to the state’s Medicaid program and another $500 million in penalties. On January 19th Johnson & Johnson reached a settlement of $158 million in a Medicaid fraud lawsuit for the state of Texas. For the company, this settlement was considered a win compared to their previous settlements and also considering the fact that the company has made billions from Risperdal. Though Johnson & Johnson lawyers took this case as a win, the company’s continuous appearance in the news has negatively impacted their sales and reputation.
—–
Statistics Show December to be one of the Deadliest Car Accident Months of the Year
With the holiday’s right around the corner, traffic is quickly picking up and winter weather is slowly moving in. Borchardt Law Firm asks all drivers to be extra cautious on the road during these hectic times. Studies have found December to be one of the deadliest months for drivers across the state of Texas. It is one of the biggest traveling months and is also prone to unpredictable weather, such as rain, sleet, snow and ice. According to the Texas Department of Transportation, in 2010 December had one of the highest reports of fatal state highway and city street accidents. Whether these accidents were a result from weather or distractions, drivers need to be aware of their surroundings and prepare for any situation. At Borchardt Law Firm, our law firm has come up with a few guidelines for drivers in hopes to help prevent accidents on the road.
Though Texas does not see snow that often, we get our fair share of rain, sleet and ice during the winter. If you must leave your house, be aware of these guidelines to help prevent you from getting into an accident.
• Before leaving your house, check your tire pressure and make sure your tires still contain enough tread to create traction on the road.
• Plan your route on main roads that have most likely already been driven on.
• Drive slowly; prepare to go under the speed limit. Give yourself at least double the amount of the time it normally takes you to get to your destination.
• You should allocate at least three times more space in between your car and the car in front of you.
• When preparing to stop gently pump your brakes, don’t slam on your breaks and hold them down – this could cause your vehicle to continuously slide.
• Keep your car in low gears to hold traction.
• Try to avoid all bridges and overpasses – they will most likely be the slickest.
• Keep your lights on, helping other cars see your vehicle.
• If you begin to hydroplane, grip your steering wheel to keep control and wait until you feel your tires contact the road.
• If storms become too severe, pull over but avoid parking under power lines, trees and other large objects that could fall on your car.
—–
FDA Vote Leads Bayer to Update Yaz Labeling
The FDA (Food and Drug Administration) continues to express concern about the potential side effects of Yaz birth control. Over the past few years health experts and the FDA have shown an increased apprehension over the once-a-day pill. Yaz, unlike older contraceptive pills, contains drospirenone, an ingredient that may be a leading factor in an increased risk in blood clots. Though all birth controls come with a risk of blood clots, studies have suggested that pills containing drospirenone slightly enhance that risk. Yaz, one of Bayers leading products, was approved in 2006. By 2008 it was the top selling birth control pill in the U.S. Due to its reputation of helping with acne and PMS symptoms millions of women became prescribed to the pill.
By 2009 Bayer’s sales for Yaz quickly began to fall. Several women filed complaints that Yaz was the leading factor in blood clots and that the Bayer did not properly inform their patients of this risk. Since then, sales of Yaz have decreased by almost 80 percent, creating a much higher threat of concern and a significant increase in lawsuits. Today, there is an estimated 4,000-6,000 patients suing Bayer for not being properly informed of the possibly severe side effects of Yaz. Blood clots can cause life threatening problems such as stroke and heart attack. There have also been reported cases of fatal blood clots. If it is a possibility that drospirenone increases the risk of what can be fatal blood clots, Bayer needs to immediately update all of Yaz’s labels to accurately inform their patients.
Though health experts still struggle to find clear evidence that drospirenone is the leading factor in an increase in blood clots the FDA panel of experts voted 21-5 that Bayer did not properly inform their patients of potential side effects. The labeling on each drug is not clearly stated and needs more detail when referring to impending severe side effects such as the increased risk of blood clots.
—–
Yaz Lawsuits Increase as Birth Controls Containing Drospirenone Create Concern
A falsely advertised Yaz commercial sparks possible health concerns for the birth control pill. Yaz, a product of Bayer Healthcare Pharmaceuticals, has received thousands of complaints from women across the U.S. about unsatisfying results after taking the pill for several months. In 2007 Yaz launched an advertisement that promoted the pill to treat severe premenstrual symptoms and mild acne, making the pill extremely desirable for most women. After the ad hit the air, millions of women switched to the pill expecting to see a positive outcome. Just two years after the launch of Yaz, their sales had jumped to 2 billion, making Yaz one of the leading birth control pills on the market and one of Bayer’s top products. However, a positive outcome wasn’t true for everyone. Many women prescribed to Yaz felt as if the advertisement was extremely misleading. Premenstrual symptoms were still happening and women with mild acne were not seeing huge improvements like the ad had emphasized.
https://www.youtube.com/watch?v=DVy-uDadBx4
As more complaints were filed the FDA, Food and Drug Administration, took a closer look at the advertisement and results of the pill. After studies found that Yaz was never actually proven to treat severe PMS symptoms, Bayer launched a corrected ad that reinstated the pill should be used “for the treatment of premenstrual dysphonic disorder, or PMDD, and moderate acne, not for the treatment of PMS or mild acne.” Though Bayer had corrected their misleading ad, the FDA still had concerns about the pill. While looking over studies the FDA noted that Yaz, unlike most birth control pills, contains the use of drospirenone, a synthetic progestin that helps prevent ovulation. Though drospirenone works well for preventing pregnancy, the FDA has shown concern that the synthetic progestin may lead to a higher risk of blood clots.
In 2008, a 24-year-old woman taking Yaz for just three months suffered from a nearly fatal blood clot that she believes also might be the cause of her blindness. The woman began taking Yaz after seeing the convincing commercial that Yaz would treat all severe PMS and mild acne symptoms. After a few months on the pill she began to feel aching pain in her legs, assuming it was simply exhaustion. Shortly after, she suffered from severe blood clots the next evening. The clots had traveled from her legs up to her lungs causing a double pulmonary embolism. As she was rushed to the hospital her heart stopped and she slipped into a coma. The 24-year-old awakened about two weeks later to realize she no longer had her vision. Doctors are not positive what exactly lead to the blood clots and the loss of vision but the woman believes it had to do with the drospirenone in the pill. (read full story)
—–
Drivers Warned About Dangers of Drowsy Driving
Drowsy driving could be the number one cause of car and truck accidents today. According to CBS News, a recent study done by the AAA Foundation for Traffic Safety, found that 96% of drivers say drowsy driving is unacceptable, however, one-third of those drivers admit to driving while drowsy in the past month. Drivers have continuously been warned about the consequences of driving while intoxicated and driving while distracted, but now, the public needs to add to the warnings about driving while drowsy.
Trucking companies across the nation have updated driving regulations with the intention to prevent drowsy driving, but whether you are driving a semi-truck across the state or a minivan to school, all drivers need to be aware of the dangers of driving drowsy. Know the warning signs to watch for when you’re behind the wheel. (read the full article)
Warning signs for drowsy driving
Trucking and car accidents are far too common on the roads today. According to the Texas Department of Transportation, in 2010 based on reportable crashes, one person was killed every 2 hours and 54 minutes, one person was injured every 2 minutes and 26 seconds, and one reportable crashed occurred every 81 seconds. These statistics are devastating. Don’t let drowsiness, intoxication or any kind of distraction be the cause of an accident. (read full statistics)
—–
Johnson & Johnson Under Pressure, Depuy Hip Implant Still on Recall
Johnson & Johnson still taking a hard hit after thousands of the Depuy artificial hips were recalled in 2010. The well known company has faced hundreds of medical device lawsuits due to the failure of the product.
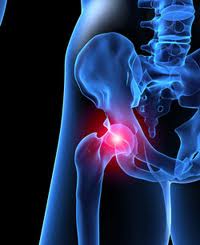
August of 2010, the DePuy ASR XL Acetabular was recalled due to unusually high rates of hip implant failure within only a few years of surgery. DePuy estimated the failure rate to be about 12-13 percent and was designed to last up to 15 years. However, results showed otherwise. Several patients ended up in constant pain resulting in several revision surgeries only a few years after the implant. Doctors continue to warn patients about the risks of all metal-on-metal hip implants. Due to the full metal design of the Depuy hip, the implant is known to shed tiny pieces of metallic ions that lead to a high risk of causing permanent tissue, muscle and bone damage. Though all orthopedic implants shed debris after a few years, the metal-on-metal poses a higher threat that can cause severe lifelong problems.
As doctors and surgeons continue to recommend other procedures of hip implants to patients rather than the metal-on-metal implants, Johnson & Johnson is striving to boost the reputation of their company. According to The New York Times, “Johnson & Johnson pleaded guilty in April 2011 to bribing European doctors and agreed to pay $70 million in fines, as a wide-ranging government investigation of corrupt overseas marketing practices by drug and device makers scored its first major victory. At least a dozen other major drug and device makers are under investigation for similar crimes.” (read full article)
—–
Nursing Home Abuse and Neglect Cases Go Unreported
Deciding when to move a loved one into a nursing home is a difficult decision. We all want the best for our family, and need to consider several things when it comes to picking the right home. Unfortunately, nursing home abuse and neglect is more common than we think.
Every year numerous nursing home abuse or neglect cases go unreported. In February of 2009, a report done by CBS News came out that showed in just two years, 1999 – 2001, “5,283 — over 30 percent — of the nursing homes in the U.S. were cited for an abuse violation. Over 2,500 of the violations were serious enough to cause actual harm or to place residents in immediate jeopardy of death or serious injury.” (read full story) At Borchardt Law Firm we wish that no family has to ever experience nursing home abuse or neglect, help prevent abuse or neglect by knowing the warning signs that you can look for when you’re loved one is in a home.
According to the Nursing Home Abuse Center signs of abuse to look for include:
Obvious signs:
• unusual bruising or bleeding
• open wounds, sores or cuts
• burns and abrasions
• sudden and unexplained change in weight
• soiling, poor hygiene, smell of urine or feces
• infections
• loss of hair
• torn, stained, or bloody clothing or bedding
Less obvious signs:
• listlessness or unresponsiveness
• infantile or other strange behaviors
• physical or emotional withdrawal
• disappearance of personal items
• sudden and unusual financial transactions
Transvaginal Mesh Lawsuit Boost FDA’s Concerns, Possible Recall for the Medical Device
While the Food and Drug Administration (FDA) determine whether to place a stronger emphasis on their safety warning about transvaginal mesh, a number of medical device litigations continue to spark concern. After receiving more than 1,500 complaints in just two years, the FDA is currently looking more closely into the serious side effects of transvaginal mesh, questioning whether the products should be pulled from the shelves.
In September 2011, a South Dakota woman filed a lawsuit against Johnson & Johnson over their vaginal mesh product, Gynecare Prolift. What was suppose to be a simple and easy procedure resulted into twelve failed surgeries and long term severe side effects. According to ABC News, after having her surgery in 2006, the patient has continuously suffered from “urinary complications, constant pain and swelling as her body continues to reject the mesh. She says she can’t sit for more than 20 minutes, she can’t have sex with her husband, and she can’t be active for more than a few minutes at a time. ‘It’s horrible. There are unknown amounts of the mesh still in me. I have extreme pain.” the woman told ABCNews.com. “I wouldn’t wish this on anyone.'”
At the time of the implantation, transvaginal mesh had not yet undergone the FDA’s standard testing for medical devices, instead the product was approved by the 510(k), a plan that “allows devices to go to market taking a short cut through the regulatory procedures as long as they are proved to be “substantially equivalent” to something already on the market”(ABC News). Johnson & Johnson had compared the Gynecare Prolift to one of their similar products, Gynemesh, which has been on the market since 2002. The patient is in the process of suing Johnson & Johnson for compensatory and punitive damages, she is also hoping to raise the awareness to women across the U.S. about the dangers of transvaginal mesh. (read full story)
—–
 Fort Worth Injury Lawyer Blog
Fort Worth Injury Lawyer Blog







